Focused Topic Networks
On behalf of its Member Societies and Patient Advocacy Groups, the European Cancer Organisation convenes interested stakeholders around ten important topics.
Led by co-chairs from our Member Societies, these Networks have been established to facilitate consensus and joint projects in our Strategy 2024-2027 The programme of the European Cancer Summit is also based on these ten topics.
In the Spotlight
In this section of our homepage, we highlight the latest range of activities that our members, patient advocates and team are working on, to support our Mission and Vision.
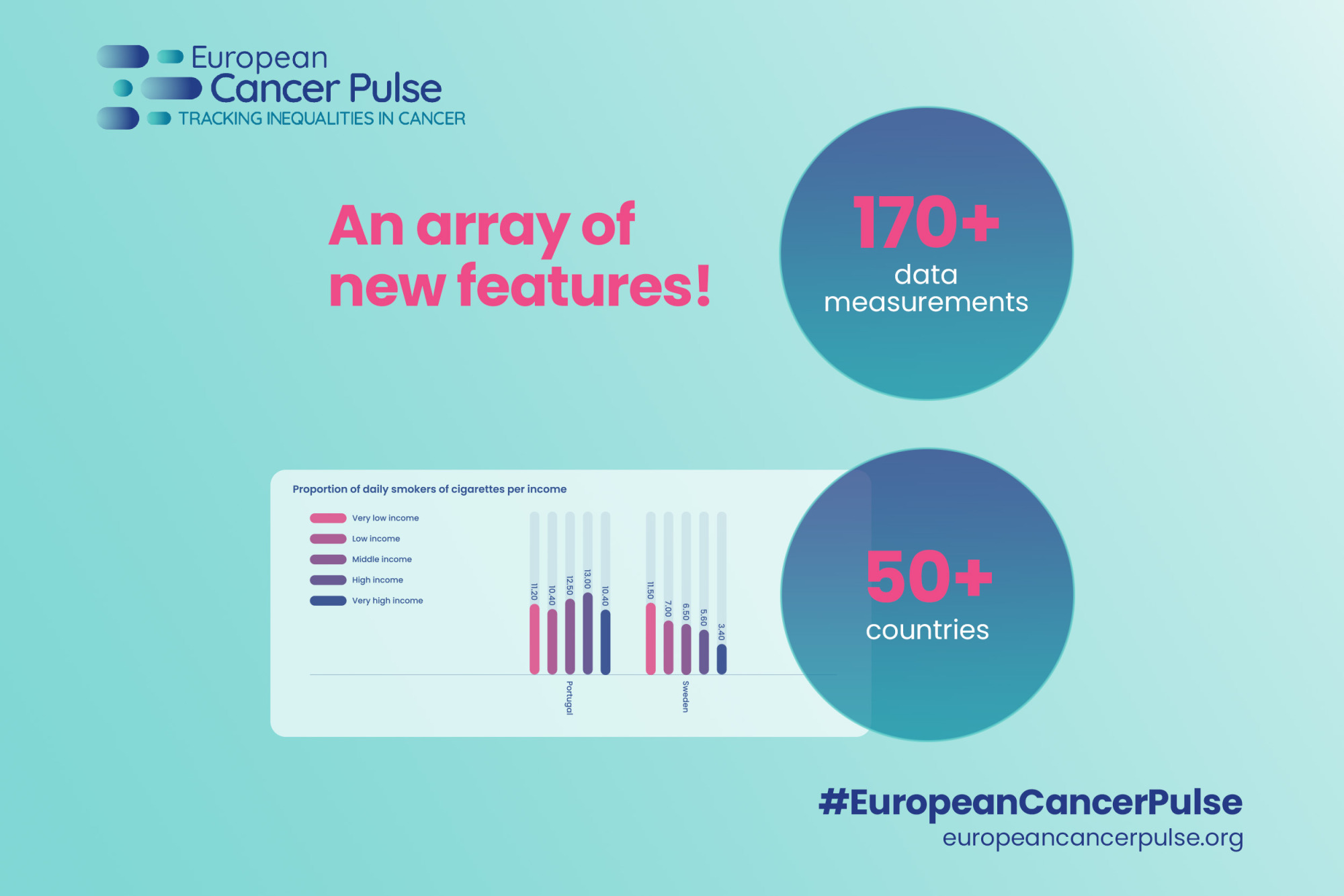
Tracking Inequalities in Cancer
The European Cancer Pulse is a unique data visualisation tool designed to track inequalities and monitor progress in cancer care across Europe, highlighting the factors that influence disparities in cancer care.
Read more-
Political Unity for Cancer Action
National & European Parliamentarians for Cancer Action brings together more than 110 relevant policymakers to design better cancer policies in Europe, learn from best practices across the cancer care continuum, and collectively create data-driven recommendations in the fight against cancer.
You can find a list of the members below.
Read more -
The European Code of Cancer Practice: Championing the Rights of Cancer Patients Across Europe
The European Cancer Organisation has launched a brand new initiative for empowering European citizens and cancer patients with ten key overarching Rights, signposting what cancer patients in Europe should expect from their healthcare system. The European Code of Cancer Practice (The Code) has been produced to assist every person with cancer across Europe to receive the best possible care, treatment, and support.
Read more
News
all news-
07 April 2024
ECO Marks World Health Day
Read more -
04 April 2024
UK Falling behind Europe in the fight against cancer
Read more -
03 April 2024
PRESS RELEASE - A Cancer Workforce in Crisis
Read more
Our Member Societies
Our 42 Member Societies are experts in their discipline or profession and elect our Board. They contribute to our Focused Topic Networks and policy approval pathway.
Patients
Receiving a cancer diagnosis is life-changing. Plans, perspectives and so much else, immediately take on different dimensions. New encounters lie ahead, which can inevitably be accompanied by a mixture of emotions, including uncertainty, fear, and a sense of lost security.